
First Comprehensive Care Plan to Prevent Preeclampsia Published in the American Journal of Obstetrics and Gynecology
SAN FRANCISCO - April 28, 2023
A new special report published in the American Journal of Obstetrics and Gynecology (AJOG) provides a methodical, comprehensive approach to managing preeclampsia, one of the most pressing issues in maternal health today, and will translate the prediction of risk into prevention of disease.
The report, “Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance and follow up,” provides specific recommendations for both expecting parents and clinicians. The Care Plan’s recommendations include consideration of daily low-dose aspirin, surveillance, behavioral strategies, patient and provider education, addressing social determinants of health, and long-term follow up. These recommendations are a significant shift from the current care approach, which has historically lacked a comprehensive, integrated strategy. While technology to reliably predict preeclampsia is imminent, until now there has not been clear direction on optimal strategies for its prevention.
A leading cause of pregnancy-related deaths in the U.S.,1 preeclampsia is a disorder of high blood pressure that can result in preterm birth, organ damage, and other severe complications during pregnancy. The impact of preeclampsia can extend across a lifetime for both moms and babies. With maternal mortality rising in recent years2 and high blood pressure disorders in pregnancy (including preeclampsia) doubling since 20073, there is an urgent need to predict, prevent, and mitigate its devastating impact.
Fortunately, there are several effective interventions that have been associated with reduced risk of preeclampsia in individuals at increased risk.4-6 The new AJOG report synthesizes them all into an objective, evidence-based recommendation for the first time and provides checklists for both individuals at risk and health care providers, which can be downloaded and shared. Today, most pregnant individuals at increased risk do not receive even one of the interventions to prevent preeclampsia. For example, less than half of high-risk patients receive low-dose aspirin.7 By streamlining the evidence-based recommendations into a straightforward Care Plan, the report systematically outlines the multi-pronged preventive approach that patients at risk should be receiving, which includes:
- Key recommendations for health care providers: Risk assessment including social determinants of health, pharmacological recommendations (including aspirin therapy and antihypertensive therapy), and behavioral recommendations (including specific information about diet, exercise, and sleep)
- Key recommendations for persons at-risk for preeclampsia:
• To discuss with a health care provider: Questions regarding aspirin use, exercise during pregnancy, blood pressure monitoring, and more
• To do on their own: Watching for signs of preeclampsia with symptoms listed, checking blood pressure at home and reporting any readings greater than 140/90, implementing dietary, exercise, and sleep changes, and more
“This new comprehensive Care Plan, developed by a diverse group of preeclampsia experts, payers and advocates is a specific and clear set of recommendations based on peer-reviewed evidence and expert opinion,” said James Roberts, M.D., a Maternal-Fetal Medicine researcher at the Magee-Womens Research Institute, UPMC and founding Principal Investigator of the Global Pregnancy Collaboration who is one of the lead authors. “The plan outlines medications, monitoring, behavioral modification, education, and considerations for social determinants of health. It is designed to be as safe, cost-effective, and practical to implement in real world practice as possible. The clear checklists further encourage their use for any pregnancy considered to be at increased risk for developing preeclampsia,” added Dr. Roberts. He said the team intends to have these checklists translated into multiple languages to further improve access and use.
”Objective prediction of preeclampsia risk months in advance will soon be possible, and the Care Plan answers the question of what to do with that information,’” said Alison Cowan, M.D., M.S.C.R. and Head of Medical Affairs at Mirvie. “An unprecedented collaboration between medical experts and preeclampsia advocates created this novel patient-centered Care Plan. It represents a critical step in preventing preeclampsia and saving lives.” Mirvie, a company developing the first platform to predict pregnancy complications by revealing the underlying biology, supported the independent development of this report to contribute to an increased understanding of how to prevent preeclampsia.
Eleni Tsigas, CEO of the Preeclampsia Foundation and one of the co-authors noted, “By centering preeclampsia survivors as experts and co-authors, alongside clinicians, researchers, and payers, we have produced a practical and proactive comprehensive Care Plan. It is written in clear and simple language, can be easily incorporated into electronic health record (EHR) systems, and is designed to be a cooperative plan for both women and their care teams. With consistent implementation in any setting, we expect this Care Plan will help address inequalities in maternal care.”
With objective, predictive testing for preeclampsia imminently on the horizon, this integrated Care Plan will translate risk prediction into disease prevention. As many women without traditional risk factors will still develop preeclampsia, this combination of novel predictive and preventive tools will prove to be indispensable for all clinicians and patients seeking to prevent this serious pregnancy complication and the lifelong health impacts of preeclampsia.
About Preeclampsia Foundation
The Preeclampsia Foundation is a U.S.-based 501(c)(3) non-profit organization established in 2000 to improve the outcomes of hypertensive disorders of pregnancy by educating, supporting, and engaging the community, improving healthcare practices, and finding a cure. We envision a world where preeclampsia and related hypertensive disorders of pregnancy no longer threaten the lives of mothers and babies. For more information, visit www.preeclampsia.org.
About Mirvie
Mirvie is shaping the future of pregnancy health by providing women, expecting parents and their doctors with an early detection window to intervene before unexpected pregnancy complications become a crisis. One in five pregnancies is impacted by complications that lead to lifelong health consequences for expecting parents and babies. The proprietary Mirvie RNA platform uses a simple blood test to reveal vital information about a pregnancy’s unique biology and detect complications months before they occur. The idea for Mirvie was sparked by the personal experience of one of the founders whose daughter was born prematurely. Mirvie’s team of world-class scientists and entrepreneurs have brought to market category-first, non-invasive tests in both women’s health and in early cancer detection, used by millions today. Founded in 2018, Mirvie has raised more than $90 million in early-stage financing from top-tier investors, including Decheng Capital, Foresite Capital, General Catalyst, GV, Khosla Ventures, and Mayfield. Mirvie is based in South San Francisco, California. To learn more about Mirvie, please visit www.mirvie.com.
About Global Pregnancy Collaboration
The Global Pregnancy Collaboration (CoLab) is an international consortium of 40 centers. CoLab promotes collaboration in pregnancy research to improve maternal and child health worldwide. This is accomplished by assisting collaboration and sharing of data and biological samples, and education with special attention to developing research infrastructure in low-resource settings. Efforts address the needs of all maternal child health care—from the simplest in the most resource-poor countries to the most sophisticated research in more privileged areas. For more information, visit https://pregnancycolab.tghn.org.
References:
- Trost SL, Beauregard J, Njie F, et al. Pregnancy-related deaths: data from Maternal Mortality Review Committees in 36 US States, 2017-2019. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services;2022.
- Gunja MZ, Gumas ED, Williams II RD. The U.S. maternal mortality crisis continues to worsen: an international comparison. The Commonwealth Fund. December 1, 2022.
- Cameron NA, Everitt I, Seegmiller LE, Yee LM, Grobman WA, Khan SS. Trends in the Incidence of New-Onset Hypertensive Disorders of Pregnancy Among Rural and Urban Areas in the United States, 2007 to 2019. J Am Heart Assoc. 2022 Jan 18;11(2):e023791. doi: 10.1161/JAHA.121.023791. Epub 2022 Jan 11. PMID: 35014858; PMCID: PMC9238536.
- US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Simon MA, Stevermer J, Tseng CW, Wong JB. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 Sep 28;326(12):1186-1191. doi: 10.1001/jama.2021.14781. PMID: 34581729.
- Woo Kinshella ML, Sarr C, Sandhu A, Bone JN, Vidler M, Moore SE, Elango R, Cormick G, Belizan JM, Hofmeyr GJ, Magee LA, von Dadelszen P; PRECISE Network. Calcium for pre-eclampsia prevention: A systematic review and network meta-analysis to guide personalised antenatal care. BJOG. 2022 Oct;129(11):1833-1843. doi: 10.1111/1471-0528.17222. Epub 2022 Jun 28. PMID: 35596262.
- Davenport MH, Ruchat SM, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, Skow RJ, Meah VL, Riske L, Sobierajski F, James M, Kathol AJ, Nuspl M, Marchand AA, Nagpal TS, Slater LG, Weeks A, Adamo KB, Davies GA, Barakat R, Mottola MF. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018 Nov;52(21):1367-1375. doi: 10.1136/bjsports-2018-099355. PMID: 30337463.
- Krishnamurti T, Davis AL, Rodriguez S, Hayani L, Bernard M, Simhan HN. Use of a Smartphone App to Explore Potential Underuse of Prophylactic Aspirin for Preeclampsia. JAMA Netw Open. 2021 Oct 1;4(10):e2130804. doi: 10.1001/jamanetworkopen.2021.30804. PMID: 34714341; PMCID: PMC8556626.
Stay tuned for more updates!
May 8 at 1 pm ET
"Strengthening hospital-based perinatal equity initiatives by partnering with community-based organizations and people with lived experience"

Related Articles

Nurses play a vital role in detecting preeclampsia and caring for patient before, during, and beyond pregnancy.

A key component needed in the fight against preeclampsia is the development of tests for simple, rapid, and accurate diagnosis and prediction through the development and adoption of biomarkers.
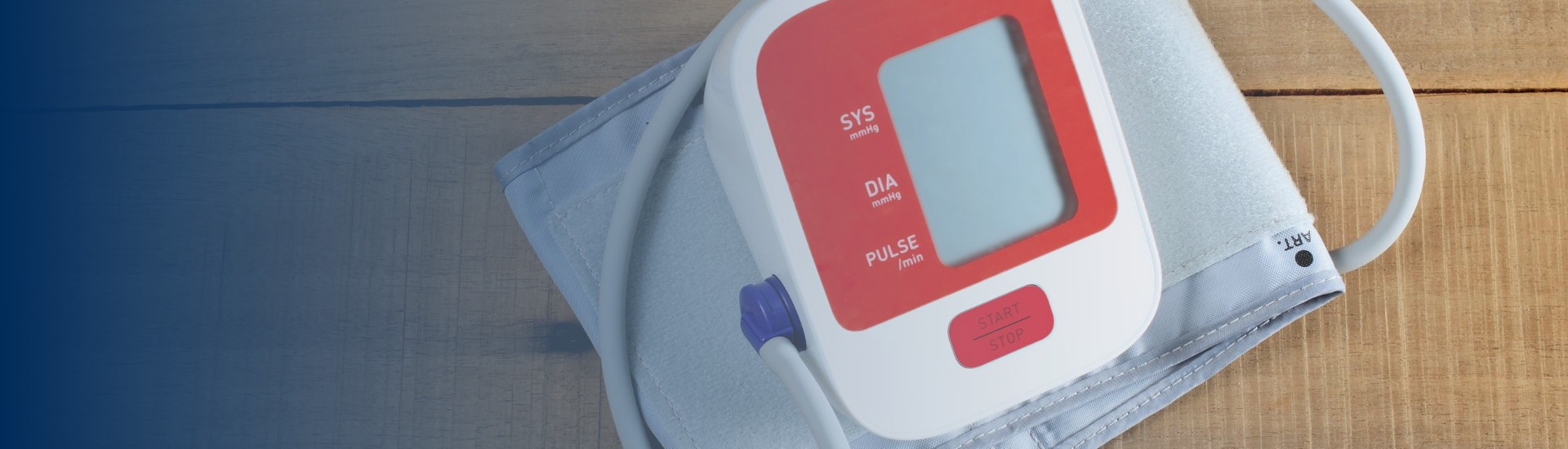
As a first step to address the need for self-monitored blood pressure, the Preeclampsia Foundation started providing the Cuff Kit™ in June 2020 to women at highest risk of developing preeclampsia and...
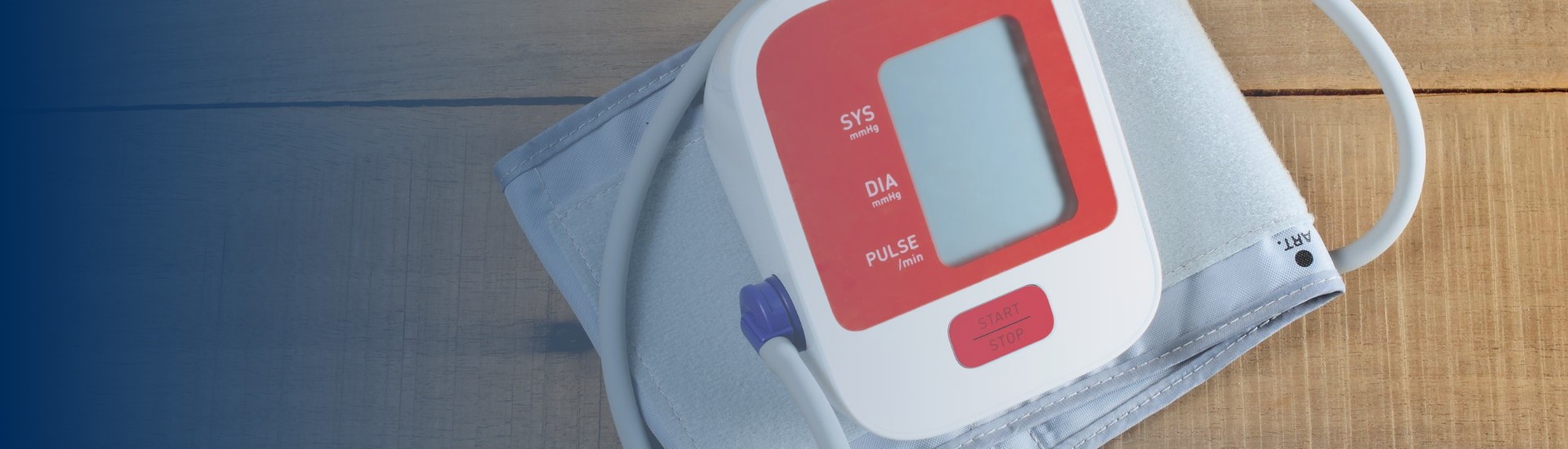
Every woman should be able to check her own blood pressure at home.
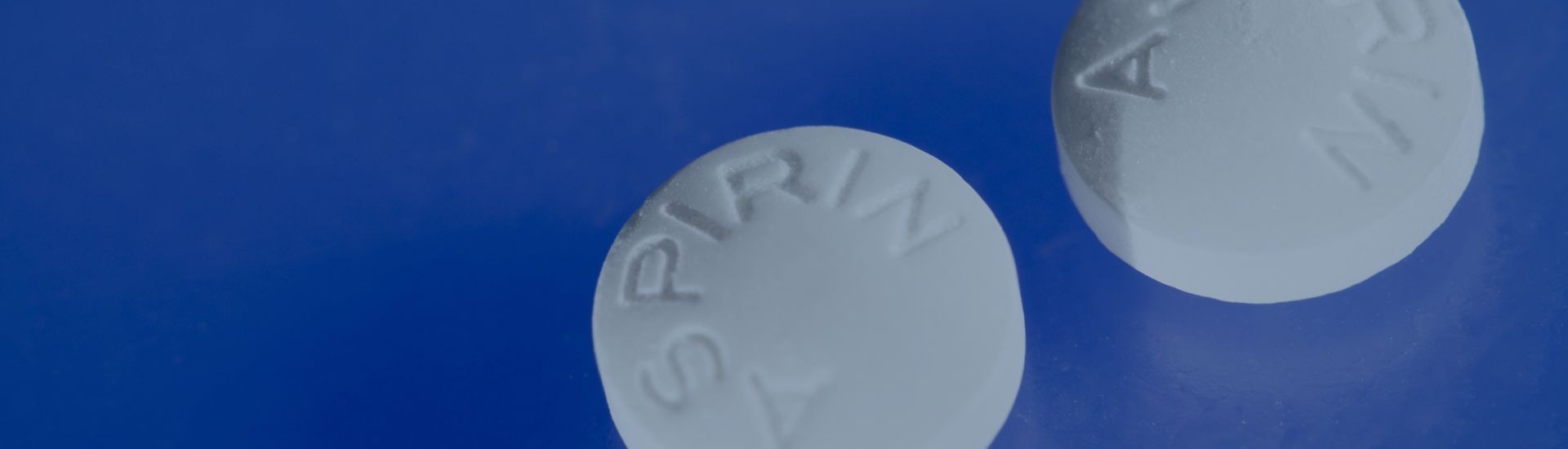
Order our Ask About Aspirin Rack Card. Aspirin can prevent the formation of blood clots. This can make aspirin useful in treating or preventing some conditions like heart attacks and st...

Washington, DC – April 11, 2024 – On April 10, one day before the start of Black Maternal Health Week, the Preeclampsia Foundation in partnership with Thermo Fisher Diagnostics held a Hill...

Recently, I came across a social media post calling attention to the global maternal health crisis from a Black woman’s perspective. Someone responded to the post asking, “What’s rac...

The Preeclampsia Foundation announced today that the Centers for Disease Control and Prevention (CDC) awarded new funding for its MoMMAs Voices program, a national coalition of patient advocacy organi...

The U.S. Preventive Services Task Force (USPSTF) released today a final recommendation statement on screening for hypertensive disorders of pregnancy. The Task Force recommends that all pregnant peopl...

September 7, 2023 - Rockville, Md. — The U.S. Department of Health and Human Services (HHS)—through the Health Resources and Services Administration (HRSA)— awarded an evaluation a...

