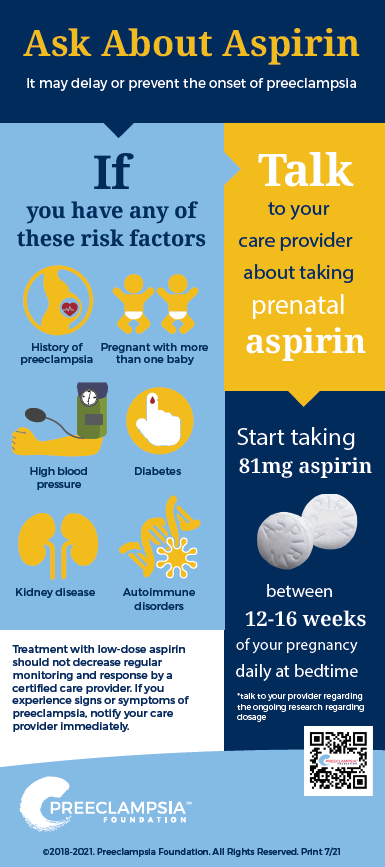
Should your patients be on prenatal aspirin?
Low-dose aspirin may delay or prevent the onset of preeclampsia
Last Updated on August 03, 2023
Order our Ask About Aspirin Rack Card.
Aspirin can prevent the formation of blood clots. This can make aspirin useful in treating or preventing some conditions like heart attacks and strokes. The dosage for aspirin can range from 50 to 6000 milligrams (mg) per day depending on the condition being treated, and aspirin may be used in combination with other medications. Low-dose aspirin ranges from 60-150 mg daily but, in the United States, the usual dose is a 81-mg tablet.
When low-dose aspirin is indicated for the prevention of preeclampsia during the prenatal period, most studies are referring to a 81-mg daily tab that is recommended by the American College of Obstetricians and Gynecologists (ACOG)[i]. Regular strength aspirin is NOT a preferred pain reliever during pregnancy.[ii]
Although there is evidence to suggest that 150 mgs may be more effective,[iii] & low-dose aspirin is generally available in the United States as 81-mg tablets, which is a reasonable dosage for prevention in women at high risk for preeclampsia. Some doctors choose to prescribe 2 81 mg tablets per day.
Who should prenatal aspirin be prescribed to?
To prevent all variations of preeclampsia including HELLP syndrome, according to the U.S. Preventive Services Task Force (USPSTF) guidelines[iv], women with one or more high-risk factors should take low-dose aspirin. Women with several moderate-risk factors may also benefit from low-dose aspirin.
| Risk Level | Risk Factors | Recommendation |
|---|---|---|
| High |
|
Recommend low-dose aspirin if the patient has one or more of these high-risk factors |
| Moderate |
|
Recommend low-dose aspirin if the patient has two or more of these moderate risk factors; consider low-dose aspirin if the patient has at least one of these moderate risk factors |
| Low |
Previous uncomplicated full-term delivery |
Do not recommend low-dose aspirin |
When should patients start taking low-dose aspirin?
Low-dose aspirin should be started between weeks 12 and 16 of pregnancy.
Although ACOG and USPSTF guidelines recommend starting between weeks 12 and 28 of your pregnancy, recent evidence shows that starting closer to the beginning of the second trimester may be more beneficial.
A review of 45 randomized trials that included over 20,000 pregnant women taking daily low-dose aspirin showed significant evidence of the prevention of preeclampsia, severe preeclampsia, and fetal growth restriction when initiated before 16 weeks’ gestation. Low-dose aspirin initiated after 16 weeks’ gestation may not be as effective at reducing the risk of preeclampsia, severe preeclampsia, and fetal growth restriction. Women at high risk for those outcomes should be identified in early pregnancy.[v]
Time of day matters.
Research shows that aspirin is most effective at bedtime when compared to morning, afternoon, and evening dose times.[vi]
What are the risks associated with taking prenatal aspirin?
As part of its assessment, the USPSTF considered whether there was any potential harm to mom or baby. Its report found:
- No increase in infant loss, growth problems, or cognition harm to the baby;
- No statistically significant impact on risk of placental abruptions, postpartum hemorrhage (bleeding), or miscarriage to the mother;
- No differences in developmental outcomes of the infants up to age 18 months.
A growing number of studies have begun to look at whether there could be long-term impacts on children. The news is hopeful: for example, one 2024 research study found that prenatal exposure to aspirin did not worsen or improve cognitive development in children between the ages of 33 and 39 months of age.[vix]
"The long-term effect of aspirin usage during preeclamptic pregnancies is actually one of the things we're collecting in The Preeclampsia Registry (www.preeclampsiaregistry.org)," said CEO Eleni Tsigas about the Preeclampsia Foundation's patient registry. "We'd like to see how offspring of women who took aspirin during their pregnancies fare many years down the road. As more patients are prescribed prenatal aspirin, research studies can help us to understand its impact."
What are the risks associated with taking prenatal aspirin?
As part of its assessment, the USPSTF considered whether there was any potential harm to mom or baby. Its report found:
- No increase in infant loss, growth problems, or cognition harm to the baby;
- No statistically significant impact on risk of placental abruptions, postpartum hemorrhage (bleeding), or miscarriage to the mother;
- No differences in developmental outcomes of the infants up to age 18 months.
Can taking low-dose aspirin increase risk of miscarriage?
Taking low doses of aspirin is not thought to increase the risk of miscarriage.[vii] Research suggests the use of aspirin during pregnancy is not associated with an increased risk of miscarriage.
Will aspirin hurt the baby?
Studies on the effects of low-dose aspirin on fetal and maternal health and development are reassuring, and low doses of aspirin administered during the first trimester do not seem to constitute risk for the fetus.[viii]
When should patients stop taking low-dose aspirin?
There are opposing arguments regarding when to discontinue aspirin treatment. Some argue that aspirin should be discontinued at 36 weeks because of the possible bleeding risks associated with delivery.
Others argue, because most preeclampsia occurs after 36 weeks, that the aspirin may be beneficial to continue through delivery, into the postpartum period.
Does taking aspirin guarantee that preeclampsia will be prevented or delayed?
Taking aspirin does not guarantee that you will not develop preeclampsia. It is simply one more thing that women can do with relative safety to reduce their overall risk. The USPSTF review took into account approximately 30,000 randomized subjects, which found a 2 to 5% risk reduction in the rate of preeclampsia. Both the USPSTF and ACOG acknowledge that tools to assess individual risk for the condition and identify subgroups of mothers most likely to benefit are still needed.
The USPSTF authors also agreed that preventing preeclampsia could reduce medical intervention in pregnancy and delivery. Preventing poor pregnancy outcomes could also reduce post-traumatic stress disorder and postpartum depression because preeclampsia is associated with poor maternal mental health outcomes.
[i] Practice Advisory on Low-Dose Aspirin and Prevention of Preeclampsia: Updated Recommendations. (2016, July 11). Retrieved from American College of Obstetricians and Gynecologists: www.acog.org
[ii] LowDose Aspirin Fact Sheet. (2016, December). Retrieved from www.mothertobaby.org: https://mothertobaby.org/fact-sheets/low-dose-aspirin/pdf/
[iii] O'Gorman N, W. D. (2016). Study protocol for the randomised controlled trial: combined mutimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE). BMJ Open.
[iv] Final Recommendation Statement: Low-Dose Aspirin for the Prevention of Morbidity and Mortality From Preeclampsia: Preventative Medicine. (2016, December). Retrieved from U.S. Preventive Services Task Force: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication#dag USPSTF recommendations were reaffirmed in 2021: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
[v] Duley, L. (2007). Antiplatelets agents for preventing pre-eclampsia and its complications. Cochran Database Syst Rev.
[vi] Hermida, et al (2005). Differing Administration Time-Dependent Effects of Aspirin on Blood Pressure in Dipper and Non-Dipper Hypertensives. Hypertension.
[vii] Kiem SA, K. M. (2006). Aspirin use and miscarriage risk. Epidemiology, 435-9.
[viii] Atallah A, e. a. (2017). Aspirin for prevention of preeclampsia. Drugs, 77:1819-1831.
[vix] Hoffman, MK, et al (2024). Neurodevelopment of Children Whose Mothers Were Randomized to Low-Dose Aspirin During Pregnancy. Obstetrics & Gynecology, 10.1097.
Related Articles

Nurses play a vital role in detecting preeclampsia and caring for patient before, during, and beyond pregnancy.

A key component needed in the fight against preeclampsia is the development of tests for simple, rapid, and accurate diagnosis and prediction through the development and adoption of biomarkers.
As a first step to address the need for self-monitored blood pressure, the Preeclampsia Foundation started providing the Cuff Kit® in June 2020 to women at highest risk of developing preeclampsia and...
Every woman should be able to check her own blood pressure at home.

Women who have had preeclampsia have three to four times the risk of high blood pressure and double the risk for heart disease and stroke: ensure they get follow-up care beyond pregnancy.

For more on the Preeclampsia Foundation's work to amplify all research related to biomarkers for improved prediction and diagnostic tools, please visit https://preeclampsia.org/biomarkers. INDIANAPOL...

GAP—SPIRIN campaign gets low-dose aspirin to those most at risk to help close the maternal health gap in preeclampsia ________ NEW YORK, January 23, 2025/PRNewswire/ – In recognition of...
1732072344.png)
While the Preeclampsia Foundation has been championing patient advocacy and representation for all families affected by hypertension in pregnancy throughout our 25 year history, we recognized the uniq...

Washington, DC – April 11, 2024 – On April 10, one day before the start of Black Maternal Health Week, the Preeclampsia Foundation in partnership with Thermo Fisher Diagnostics held a Hill...

Recently, I came across a social media post calling attention to the global maternal health crisis from a Black woman’s perspective. Someone responded to the post asking, “What’s rac...